金刚石以优异的性能在力学、光学、热学和电子学(如半导体)等领域发挥着重要作用。然而,金刚石表面质量会影响其在这些领域的应用效果,因此通过高效抛光技术获得高质量表面一直是金刚石研究的重点内容。金刚石抛光技术主要有机械抛光、热化学抛光、激光抛光和化学机械抛光等,其中化学机械抛光(CMP)具有设备运行成本低、工艺简单、抛光后表面损伤小等优点。
近日,北方工业大学安康副教授联合北京科技大学李成明研究团队在《人工晶体学报》2024年第10期发表了题为《金刚石化学机械抛光研究进展》的综述论文(第一作者:安康;通信作者:刘峰斌、李成明)。该论文在对几种抛光方法进行分析对比的基础上,聚焦于CMP领域,对其发展历程进行了较详尽的对比与分析。早期CMP技术虽在工艺和抛光效率上存在一定局限,但为后续技术的创新与优化奠定了基础;H2O2及其混合物的应用,不仅增强了CMP过程中的化学反应活性,提高了材料去除率,还有效降低了表面粗糙度,改善了金刚石表面质量;光催化辅助化学机械抛光可使金刚石达到高表面质量,但设备相对复杂,无法满足大规模生产的需求,需要进一步研究和优化。此外,本文还对化学机械抛光的未来发展进行了预测,为相关领域研究人员提供参考。
论文题录●●
安康, 许光宇, 吴海平, 张亚琛, 张永康, 李利军, 李鸿, 张旭芳, 刘峰斌, 李成明. 金刚石化学机械抛光研究进展[J]. 人工晶体学报, 2024, 53(10): 1675-1687.
AN Kang, XU Guangyu, WU Haiping, ZHANG Yachen, ZHANG Yongkang, LI Lijun, LI Hong, ZHANG Xufang, LIU Fengbin, LI Chengming. Research Progress in Chemical Mechanical Polishing of Diamond[J]. Journal of Synthetic Crystals, 2024, 53(10): 1675-1687.
章节结构
0 引言
1 金刚石的抛光方法
2 金刚石的化学机械抛光
2.1 早期化学机械抛光
2.2 H2O2及其混合物化学机械抛光
2.3 光催化辅助化学机械抛光
3 金刚石化学机械抛光的原子级去除机理研究
4 结语与展望
文章导读
当前,金刚石的表面抛光技术主要有机械抛光、热化学抛光、激光抛光(laser polishing, LP)、离子束抛光(ion beam polishing, IBP)、等离子体辅助抛光(plasma assisted polishing, PAP)和CMP等。
机械抛光是最常用的金刚石抛光方法,抛光示意图如图1所示。抛光时,含有金刚石粉的抛光盘高速旋转,通过样品台向下施加压力,实现材料的去除。机械抛光会造成金刚石工件的表面损伤和亚表面损伤,抛光过程中的机械冲击会导致抛光表面形成凹坑、亚表面裂纹和晶格损伤,亚表面裂纹和晶格损伤无法通过后续的抛光步骤消除,且难以被光学设备检测。

图1 机械抛光装置示意图
热化学抛光是以碳原子在热金属中的扩散、金刚石转化为石墨和金刚石的氧化为基础的抛光技术;热化学抛光时,金刚石膜在真空、氢气或惰性气体气氛下,在加热到750~1000 ℃的热铁光盘上转动摩擦,在高温条件下,通过碳原子向铁质抛光盘扩散来实现金刚石膜的平整化;激光抛光通过激光束照射到金刚石厚膜表面,使金刚石膜表面温度升高,进而使被加热的金刚石表面碳原子气化和石墨化,达到去除材料的目的;离子束抛光利用氧或具有较大溅射率的惰性气体(Ar)离子,对金刚石膜进行溅射刻蚀,可实现精细化抛光;等离子体辅助抛光同样用于金刚石精细化抛光,可提高金刚石表面光洁度,Yamamura等[41]采用PAP技术对CVD单晶金刚石进行抛光,先使用含水蒸气的氩基等离子体对抛光板和单晶金刚石(100)表面进行改性,后在10~52.6 kPa的抛光压力下对单晶金刚石进行抛光,结果表明,抛光后的表面粗糙度为0.13 nm,如图2所示。

图2 等离子体辅助抛光后的单晶金刚石(100)面AFM照片[41]
激光抛光、离子束抛光和等离子体辅助抛光在粗抛和精抛领域有着各自的优势,但设备的使用成本明显高于其他抛光方法。常见金刚石抛光特点如表1所示。
表1 常见金刚石抛光方法特点
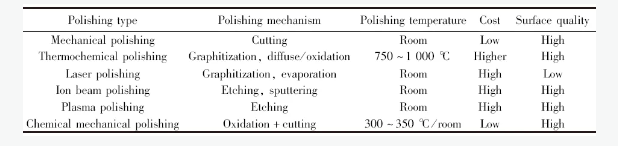
化学机械抛光(见图3)是一种超精密抛光的加工方法,通过在机械抛光过程中加入氧化剂,氧化碳原子提高抛光速率,并且具有表面损伤小、粗糙度低、设备简单、运行维护成本低,抛光后的表面污染较轻等优点,在金刚石抛光领域逐渐受到重视。

图3 化学机械抛光装置示意图[43]
早期化学机械抛光以高温熔融盐作为氧化剂进行抛光。Yuan等[49]为了降低环境污染,选用K2FeO4作为氧化剂,在化学机械抛光前用不同尺寸的金刚石粉进行机械研磨,使CVD金刚石膜的表面粗糙度降至1.042 nm,之后通过在K2FeO4加入金刚石、B4C、SiC、Al2O3等四种磨料配制抛光液,用H3PO4和NaOH来调节抛光液的pH值,抛光温度为50 ℃。抛光结果表明,获得的金刚石表面粗糙度为0.478 nm,无表面划痕或凹坑,如图4所示。

图4 金刚石表面光学图像[49]。(a)机械抛光;(b)化学机械抛光
为了验证不同种类的氧化剂对化学机械抛光的影响,Yuan等[50]开始进行对比试验,用金刚石粉进行机械抛光后,表面粗糙度下降到48 nm,再用K2FeO4、KIO4、KMnO4和K2CrO7等不同氧化剂在50 ℃的条件下,进行10组化学机械抛光对比试验,抛光机理如图5所示,其中在化学机械抛光过程中使用K2FeO4抛光出的金刚石表面粗糙度为8.72 nm,具有最佳的表面质量。

图5 化学机械抛光机理示意图[50]
H2O2是一种强氧化剂,使用H2O2溶液作为抛光液,在室温下进行化学机械抛光后,可得到原子级光滑的表面。Kubota等[54]随后改进了工艺,通过在H2O2溶液中用铁板对金刚石进行化学机械抛光,设置工作台和样品台的转速均为40 r/min,压力为3.3 MPa,抛光15 h后,在3 μm×3 μm的面积上,得到Ra=0.784 nm,RMS=1.076 nm的表面,而在500 nm×500 nm的面积上可得到表面粗糙度为0.170 nm,RMS=0.310 nm。反应机理如图6所示,在Fe2+的催化下,H2O2会分解成具有强氧化性的·OH,在金刚石表面形成C=O和C—OH等化学键,磨料会在压力的作用下与金刚石表面的C=O和C—OH结合,在剪切力作用下,金刚石表面的C—C键会发生断裂,从而实现C原子去除。

图6 基于Fenton反应的化学机械抛光过程中碳原子的去除机理示意图[55]
为了验证Fe2+对抛光的影响,Yuan等[57]采用机械研磨和化学机械抛光相结合的方法,利用磨料颗粒和过渡金属离子进行室温抛光。先进行机械研磨,得到粗糙度约为0.2 μm的金刚石表面。配制质量分数为30%的H2O2溶液100 g、去离子水100 g、W0.5金刚石粉10 g、FeSO4水溶液100 g的抛光液,用于化学机械抛光处理,抛光时间为3 h,获得表面粗糙度0.452 nm的超精密光滑金刚石表面。通过对比实验,发现相同条件下不含Fe2+的抛光液抛光出的金刚石表面粗糙度为0.741 nm,证明了Fe2+的存在增强了抛光效果,如图7所示。

图7 金刚石表面显微干涉图像[57]
Yuan等[55]对比了几种基于Fenton反应的抛光液对金刚石化学机械抛光的影响,分别是FeSO4+H2O2、Fe2(SO4)3+H2O2和Fe·OH+H2O2,结果表明用Fe2(SO4)3+H2O2试剂抛光金刚石,在868 μm×868 μm范围内,可得到最低的表面粗糙度0.076 nm,去除率最高可达752 nm/h。Fe2(SO4)3+H2O2作为抛光剂抛光效果最好的原因是H2O2被快速消耗,金刚石不能被完全氧化,而Fe3+需要消耗H2O2生成Fe2+,然后生成·OH,反应速率较慢,因此能够对金刚石长时间氧化(见图8)。

图8 氧化前、后金刚石表面SEM照片[55]
金刚石的带隙能为5.45 eV,可以在波长小于225 nm的紫外照射下激发产生空穴和电子对,并立即与大气中的氧和水分子结合,成键反应产生大量的O原子和·OH,使金刚石表面氧化。研究人员基于这一理论,提出了光催化辅助化学机械抛光(photocatalytic assisted chemical mechanical polishing, PCMP)法,Anan等[60]用紫外光(UV)辐照抛光单晶金刚石(见图9),用石英抛光盘对Ib型单晶金刚石进行抛光,紫外光可以透过石英抛光盘照射在金刚石表面。抛光前在235 nm×309 nm范围内样品的表面粗糙度为1.35 nm,经过2 h的UV抛光,样品表面粗糙度达到0.19 nm,而非UV照射抛光的金刚石表面粗糙度仅为0.74 nm,并在单晶金刚石的(100)面和(110)面均证实了紫外辐照的有效性。

图9 高速水平主轴紫外线抛光机[60]
在这一基础上,有学者将光催化辅助CMP与Fenton反应相结合,用于提高金刚石表面质量,Yang等[63]以纳米金刚石(nanodiamond, ND)和CuFe层状双氢氧化物(layered double hydroxide, LDH)为原料制备了用于可见光-Fenton反应的催化剂,将质量分数为20%的H2O2溶液混合ND/LDH催化剂作为抛光液,在抛光过程中开启可见光源,ND/LDH催化剂在可见光照射下,发生Fenton反应产生具有强氧化性的·OH,单晶金刚石表面的碳原子经过·OH氧化,再经过机械作用的处理,得到光滑的表面,实验装置如图10所示。
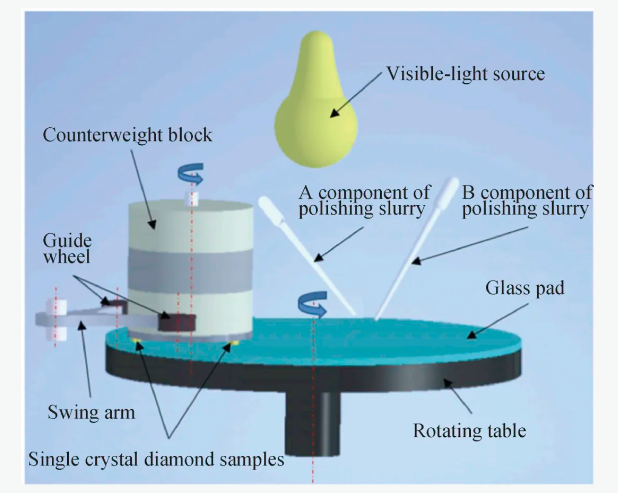
图10 实验装置示意图[63]
纳米TiO2在受到一定波长(387.5 nm)的紫外光照射后,处于价带的电子就会被激发跃迁到导带上,同时在价带上产生带正电的空穴,空穴可以将吸附在TiO2颗粒表面的OH-和H2O氧化,生成具有强氧化性的·OH,原理如图11所示。
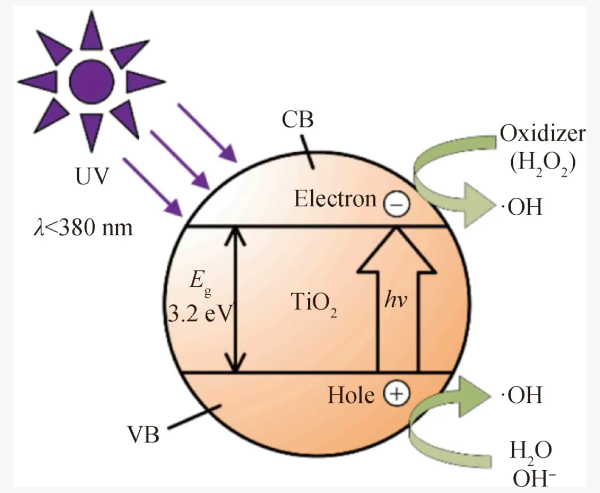
图11 TiO2紫外光催化反应的原理[66]
光催化辅助化学机械抛光可提高金刚石表面质量,达到纳米级粗糙度。但相比传统的化学机械抛光技术,设备复杂度较高,无法满足大规模生产的需求,需要进一步地研究和优化,以提高其实际应用能力。表2对化学机械抛光常用氧化剂成分进行了总结。
表2 文献中化学机械抛光常用氧化剂成分总结

分子动力学(molecular dynamics, MD)模拟可以通过高时间和空间分辨率可视化材料去除的细节,是一种适合研究原子级材料去除机理的方法。Yang等[77]和Liu等[78]均采用分子动力学方法研究了金刚石在金刚石(100)面上摩擦滑动时,金刚石晶体表层非晶态碳的形成和演化过程,模型如图12所示,模拟结果表明,在摩擦作用下,金刚石sp3杂化碳向sp2杂化发生转变,沿[100]方向滑动的非晶化速率大于沿[110]方向滑动的非晶化速率。
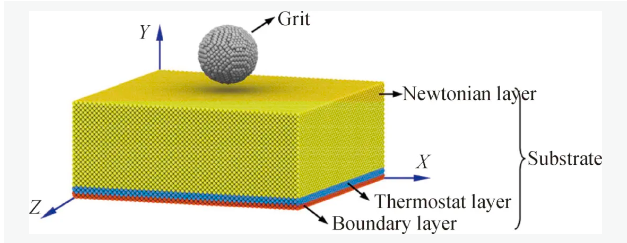
图12 金刚石晶体机械抛光MD模型[78]
Guo等[58]用分子动力学模拟研究了SiO2磨料在H2O2/纯H2O水溶液中化学机械抛光金刚石过程中的原子去除机理,模型如图13所示。金刚石表面的C与·OH、O和H结合成为C—OH、C—O、C—H键,在机械抛光的作用下,金刚石表面的C原子以CO、CO2或C链的形式被快速去除,从原子尺度上解释了化学机械抛光过程中C原子的去除过程,并进一步研究压力对材料去除率的影响,发现压力越大金刚石表面吸附的·OH越多,越有利于C原子的去除。

图13 SiO2磨料在H2O2/纯H2O水溶液中抛光过程MD示意图[58]
结语与展望
当前金刚石正以每年数亿美元的市场规模扩大应用范围,表面质量是影响其应用的重要因素。已有多种抛光技术应用于金刚石平整化过程,本综述对其优缺点进行了总结分析。化学机械抛光具有较高去除率、高表面质量、低加工成本等优势,是一种高效的抛光方法,尤其是H2O2及相关加工方法的使用,不仅使金刚石表面粗糙度达到亚纳米级,可以获得超光滑且低损伤的表面,而且降低了化学污染。未来,实现金刚石大面积、无亚表面损伤的抛光依旧是其在半导体、热沉等领域获得应用的重要基础。
参考文献
[1]王小安, 汪建华, 吕琳, 等. 高浓度氩气对金刚石膜的质量、晶粒尺寸和硬度的影响[J]. 金刚石与磨料磨具工程, 2015, 35(5): 20-24.
WANG X A, WANG J H, LV L, et al. Effect of high Ar concentration on quality, grain size and hardness of diamond films[J]. Diamond & Abrasives Engineering, 2015, 35(5): 20-24 (in Chinese).
[2]KHABASHESKU V, FILONENKO V, BAGRAMOV R, et al. Nanoengineered polycrystalline diamond composites with advanced wear resistance and thermal stability[J]. ACS Applied Materials & Interfaces, 2021, 13(49): 59560-59566.
[3]WANG L, BAI G, LI N, et al. Unveiling interfacial structure and improving thermal conductivity of Cu/diamond composites reinforced with Zr-coated diamond particles[J]. Vacuum, 2022, 202: 111133.
[4]WILDI T, KISS M, QUACK N. Diffractive optical elements in single crystal diamond[J]. Optics Letters, 2020, 45(13): 3458.
[5]ZHUANG G L, ZONG W J, TANG Y F, et al. Crystal orientation and material type related suppression to the graphitization wear of micro diamond tool[J]. Diamond and Related Materials, 2022, 127: 109182.
[6]HAO X B, LIU B J, LI Y C, et al. Diamond single crystal-polycrystalline hybrid microchannel heat sink strategy for directional heat dissipation of hot spots in power devices[J]. Diamond and Related Materials, 2023, 135: 109858.
[7]赵正平. 超宽禁带半导体金刚石功率电子学研究的新进展[J]. 半导体技术, 2021, 46(1): 1-14.
ZHAO Z P. New research progress in ultra wide bandgap semiconductor diamond power electronics[J]. Semiconductor Technology, 2021, 46(1): 1-14 (in Chinese).
[8]ROY S, BALLA V K, MALLIK A K, et al. A comprehensive study of mechanical and chemo-mechanical polishing of CVD diamond[J]. Materials Today: Proceedings, 2018, 5(3): 9846-9854.
[9]CHENG C Y, TSAI H Y, WU C H, et al. An oxidation enhanced mechanical polishing technique for CVD diamond films[J]. Diamond and Related Materials, 2005, 14(3): 622-625.
[10]LUO H, AJMAL K M, LIU W, et al. Polishing and planarization of single crystal diamonds: state-of-the-art and perspectives[J]. International Journal of Extreme Manufacturing, 2021, 3(2): 22003.
[11]SCHRECK M, GSELL S, BRESCIA R, et al. Ion bombardment induced buried lateral growth: the key mechanism for the synthesis of single crystal diamond wafers[J]. Scientific Reports, 2017, 7: 44462.
[12]吴百融, 薛常喜. 机械研磨单晶金刚石刀具前刀面精度[J]. 金刚石与磨料磨具工程, 2019, 39(2): 21-25.
WU B R, XUE C X. Precision of rake face of mechanically lapped single crystal diamond tool[J]. Diamond & Abrasives Engineering, 2019, 39(2): 21-25 (in Chinese).
[13]LEE Y C, LIN S J, BUCK V, et al. Surface acoustic wave properties of natural smooth ultra-nanocrystalline diamond characterized by laser-induced SAW pulse technique[J]. Diamond and Related Materials, 2008, 17(4): 446-450.
[14]MATSUMAE T, KURASHIMA Y, UMEZAWA H, et al. Room-temperature bonding of single-crystal diamond and Si using Au/Au atomic diffusion bonding in atmospheric air[J]. Microelectronic Engineering, 2018, 195: 68-73.
[15]严朝辉, 汪建华, 满卫东, 等. CVD金刚石厚膜的机械抛光研究[J]. 金刚石与磨料磨具工程, 2007, 27(3): 32-35.
YAN Z H, WANG J H, MAN W D, et al. Study of mechanical polishing of CVD diamond thick films[J]. Diamond & Abrasives Engineering, 2007, 27(3): 32-35 (in Chinese).
[16]TANG C J, NEVES A J, FERNANDES A J S, et al. A new elegant technique for polishing CVD diamond films[J]. Diamond and Related Materials, 2003, 12(8): 1411-1416.
[17]WEIMA J A, VON BORANY J, GROTZSCHEL R, et al. Investigating contaminants on thermochemically refined surfaces of chemical vapor deposited diamond films[J]. Journal of the Electrochemical Society, 2002, 149(5): G301.
[18]ZONG W J, ZHANG J J, LIU Y, et al. Achieving ultra-hard surface of mechanically polished diamond crystal by thermo-chemical refinement[J]. Applied Surface Science, 2014, 316: 617-624.
[19]OZKAN A M, MALSHE A P, BROWN W D. Sequential multiple-laser-assisted polishing of free-standing CVD diamond substrates[J]. Diamond and Related Materials, 1997, 6(12): 1789-1798.
[20]YOSHIDA A, DEGUCHI M, KITABATAKE M, et al. Atomic level smoothing of CVD diamond films by gas cluster ion beam etching[J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 1996, 112: 248-251.
[21]GROGAN D F, ZHAO T, BOVARD B G, et al. Planarizing technique for ion-beam polishing of diamond films[J]. Applied Optics, 1992, 31(10): 1483-1487.
[22]DU C Y, DAI Y F, GUAN C L, et al. High efficiency removal of single point diamond turning marks on aluminum surface by combination of ion beam sputtering and smoothing polishing[J]. Optics Express, 2021, 29(3): 3738-3753.
[23]LIU N, SUGAWARA K, YOSHITAKA N, et al. Damage-free highly efficient plasma-assisted polishing of a 20-mm square large mosaic single crystal diamond substrate[J]. Scientific Reports, 2020, 10: 19432.
[24]LUO H, AJMAL K M, LIU W, et al. Atomic-scale and damage-free polishing of single crystal diamond enhanced by atmospheric pressure inductively coupled plasma[J]. Carbon, 2021, 182: 175-184.
[25]HAISMA J, VAN DER KRUIS F J H M, SPIERINGS B A C M, et al. Damage-free tribochemical polishing of diamond at room temperature: a finishing technology[J]. Precision Engineering, 1992, 14(1): 20-27.
[26]HITCHINER M P, WILKS E M, WILKS J. The polishing of diamond and diamond composite materials[J]. Wear, 1984, 94(1): 103-120.
[27]HARRIS D C. Materials for infrared windows and domes: properties and performance[Z]. Portland: Copyright Clearance Center, 2000: 24.
[28]ZHENG Y T, YE H T, THORNTON R, et al. Subsurface cleavage of diamond after high-speed three-dimensional dynamic friction polishing[J]. Diamond and Related Materials, 2020, 101: 107600.
[29]LIANG Y F, ZHENG Y T, WEI J J, et al. Effect of grain boundary on polycrystalline diamond polishing by high-speed dynamic friction[J]. Diamond and Related Materials, 2021, 117: 108461.
[30]徐锋, 左敦稳, 王珉, 等. CVD金刚石厚膜的机械抛光及其残余应力的分析[J]. 人工晶体学报, 2004, 33(3): 436-440.
XU F, ZUO D W, WANG M, et al. Study on mechanical polishing for CVD diamond thick film and its residual stresses[J]. Journal of Synthetic Crystals, 2004, 33(3): 436-440 (in Chinese).
[31]KUBOTA A, NAGAE S, MOTOYAMA S. High-precision mechanical polishing method for diamond substrate using micron-sized diamond abrasive grains[J]. Diamond and Related Materials, 2020, 101: 107644.
[32]AN K, LIU P, ZHANG Y K, et al. Prestressing method to inhibit crack initiation and expansion in a large-sized diamond film during polishing[J]. Diamond and Related Materials, 2024, 144: 111022.
[33]刘敬明, 蒋政, 张恒大, 等. 大面积CVD金刚石膜的热铁板抛光[J]. 北京科技大学学报, 2001, 23(1): 42-44.
LIU J M, JIANG Z, ZHANG H D, et al. Thermo-chemical polishing of large area CVD diamond films[J]. Chinese Journal of Engineering, 2001, 23(1): 42-44 (in Chinese).
[34]王德政, 周克崧, 韩培刚, 等. 金刚石薄膜机械抛光的研究[J]. 广东有色金属学报, 1997(1): 43-46.
WANG D Z, ZHOU K S, HAN P G, et al. Research on mechanical polishing of diamond films[J]. Journal of Guangdong Nonferrous Metals, 1997(1): 43-46(in Chinese).
[35]ZAITSEV A M, KOSACA G, RICHARZ B, et al. Thermochemical polishing of CVD diamond films[J]. Diamond and Related Materials, 1998, 7(8): 1108-1117.
[36]马泳涛, 张建立, 李钝, 等. 热铁盘法抛光CVD金刚石的微观表面研究[J]. 金刚石与磨料磨具工程, 2008, 28(4): 57-61+65.
MA Y T, ZHANG J L, LI D, et al. Study on micro surfaces of CVD diamond polished by hot metal plate[J]. Diamond & Abrasives Engineering, 2008, 28(4): 57-61+65 (in Chinese).
[37]PRIESKE M, VOLLERTSEN F. Picosecond-laser polishing of CVD-diamond coatings without graphite formation[J]. Materials Today: Proceedings, 2021, 40: 1-4.
[38]KOMLENOK M, PASHININ V, SEDOV V, et al. Femtosecond and nanosecond laser polishing of rough polycrystalline diamond[J]. Laser Physics, 2022, 32(8): 084003.
[39]NAGASE T, KATO H, PAHLOVY S A, et al. Nanosmoothing of single crystal diamond chips by 1 keV Ar+ ion bombardment[J]. Journal of Vacuum Science & Technology B, 2010, 28(2): 263-267.
[40]MI S C, TOROS A, GRAZIOSI T, et al. Non-contact polishing of single crystal diamond by ion beam etching[J]. Diamond and Related Materials, 2019, 92: 248-252.
[41]YAMAMURA K, EMORI K, SUN R, et al. Damage-free highly efficient polishing of single-crystal diamond wafer by plasma-assisted polishing[J]. CIRP Annals, 2018, 67(1): 353-356.
[42]ZHENG Y T, JIA Y W, LIU J L, et al. Surface etching evolution of mechanically polished single crystal diamond with subsurface cleavage in microwave hydrogen plasma: topography, state and electrical properties[J]. Vacuum, 2022, 199: 110932.
[43]XIAO C, HSIA F C, SUTTON-COOK A, et al. Polishing of polycrystalline diamond using synergies between chemical and mechanical inputs: a review of mechanisms and processes[J]. Carbon, 2022, 196: 29-48.
[44]THORNTON A G, WILKS J. The polishing of diamonds in the presence of oxidising agents[J]. Diamond and Related Materials, 1974(39):39-42.
[45]KUHNLE J, WEIS O. Mechanochemical superpolishing of diamond using NaNO3 or KNO3 as oxidizing agents[J]. Surface Science, 1995, 340: 16-22.
[46]OLLISON C D, BROWN W D, MALSHE A P, et al. A comparison of mechanical lapping versus chemical-assisted mechanical polishing and planarization of chemical vapor deposited (CVD) diamond[J]. Diamond and Related Materials, 1999, 8(6): 1083-1090.
[47]WANG C Y, ZHANG F L, KUANG T C, et al. Chemical/mechanical polishing of diamond films assisted by molten mixture of LiNO3 and KNO3[J]. Thin Solid Films, 2006, 496(2): 698-702.
[48]REN J, ZHANG K L, WANG F, et al. Investigation of diamond films polished by thermal chemical mechanical polishing[J]. ECS Transactions, 2013, 52(1): 517.
[49]YUAN Z W, ZHENG P, WEN Q, et al. Chemical kinetics mechanism for chemical mechanical polishing diamond and its related hard-inert materials[J]. The International Journal of Advanced Manufacturing Technology, 2018, 95(5): 1715-1727.
[50]YUAN Z W, JIN Z J, ZHANG Y J, et al. Chemical mechanical polishing slurries for chemically vapor-deposited diamond films[J]. Journal of Manufacturing Science and Engineering, 2013, 135(4): 041006.
[51]TOKUDA N, TAKEUCHI D, RI S G, et al. Flattening of oxidized diamond (111) surfaces with H2SO4/H2O2 solutions[J]. Diamond and Related Materials, 2009, 18: 213-215.
[52]KUBOTA A, FUKUYAMA S, ICHIMORI Y, et al. Surface smoothing of single-crystal diamond (100) substrate by polishing technique[J]. Diamond and Related Materials, 2012, 24: 59-62.
[53]KUBOTA A, NAGAE S, MOTOYAMA S, et al. Two-step polishing technique for single crystal diamond (100) substrate utilizing a chemical reaction with iron plate[J]. Diamond and Related Materials, 2015, 60: 75-80.
[54]KUBOTA A, MOTOYAMA S, TOUGE M. Surface smoothing of a polycrystalline diamond using an iron plate-H2O2 chemical reaction[J]. Diamond and Related Materials, 2016, 69: 96-101.
[55]YUAN S, GUO X G, LI M, et al. An insight into polishing slurry for high quality and efficiency polishing of diamond[J]. Tribology International, 2022, 174: 107789.
[56]KUBOTA A, NAGAE S, TOUGE M. Improvement of material removal rate of single-crystal diamond by polishing using H2O2 solution[J]. Diamond and Related Materials, 2016, 70: 39-45.
[57]YUAN S, GUO X G, HUANG J X, et al. Sub-nanoscale polishing of single crystal diamond (100) and the chemical behavior of nanoparticles during the polishing process[J]. Diamond and Related Materials, 2019, 100: 107528.
[58]GUO X G, YUAN S, WANG X L, et al. Atomistic mechanisms of chemical mechanical polishing of diamond (100) in aqueous H2O2/pure H2O: Molecular dynamics simulations using reactive force field (ReaxFF)[J]. Computational Materials Science, 2019, 157: 99-106.
[59]LIAO L X, LUO S M, CHANG X F, et al. Study on the mechanism of chemical mechanical polishing on high-quality surface of single crystal diamond[J]. Journal of Manufacturing Processes, 2023, 105: 386-398.
[60]ANAN S, TOUGE M, KUBOTA A, et al. Study on ultra precision polishing of single crystal diamond substrates under ultraviolet irradiation[J]. Key Engineering Materials, 2009, 407/408: 355-358.
[61]WATANABE J, TOUGE M, SAKAMOTO T. Ultraviolet-irradiated precision polishing of diamond and its related materials[J]. Diamond and Related Materials, 2013, 39: 14-19.
[62]KUBOTA A, TAKITA T. Novel planarization method of single-crystal diamond using 172 nm vacuum-ultraviolet light[J]. Precision Engineering, 2018, 54: 269-275.
[63]YANG H P, JIN Z J, NIU L, et al. Visible-light catalyzed assisted chemical mechanical polishing of single crystal diamond[J]. Diamond and Related Materials, 2022, 125: 108982.
[64]YANG H P, JIN Z J, NIU H, et al. A novel visible-light catalyzed assisted single crystal diamond chemical mechanical polishing slurry and polishing mechanism[J]. Materials Today Communications, 2022, 33: 104249.
[65]高濂, 郑珊, 张青红, 等. 纳米氧化钛光催化材料及应用[M]. 北京: 化学工业出版社, 2002.
GAO L, ZHENG S, ZHANG Q H, et al. Nanometer titanium oxide photocatalytic materials and their applications[M]. Beijing: Chemical Industry Press, 2002 (in Chinese).
[66]LIU W T, XIONG Q, LU J B, et al. Tribological behavior of single crystal diamond based on UV photocatalytic reaction[J]. Tribology International, 2022, 175: 107806.
[67]苑泽伟, 杜海洋, 何艳, 等. 光催化辅助化学机械抛光CVD金刚石抛光液的研制[J]. 金刚石与磨料磨具工程, 2016, 36(5): 15-20.
YUAN Z W, DU H Y, HE Y, et al. Preparation of slurry for photocatalytic assisted chemical mechanical polishing CVD diamond[J]. Diamond & Abrasives Engineering, 2016, 36(5): 15-20 (in Chinese).
[68]SHAO J Y, ZHAO Y J, ZHU J H, et al. A new slurry for photocatalysis-assisted chemical mechanical polishing of monocrystal diamond[J]. Machines, 2023, 11(6): 664.
[69]HAN X S, HU Y Z, YU S Y. Investigation of material removal mechanism of silicon wafer in the chemical mechanical polishing process using molecular dynamics simulation method[J]. Applied Physics A, 2009, 95(3): 899-905.
[70]HARRISON J A, WHITE C T, COLTON R J, et al. Molecular-dynamics simulations of atomic-scale friction of diamond surfaces[J]. Physical Review B, 1992, 46(15): 9700-9708.
[71]HARRISON J A, WHITE C T, COLTON R J, et al. Effects of chemically bound, flexible hydrocarbon species on the frictional properties of diamond surfaces[J]. The Journal of Physical Chemistry, 1993, 97(25): 6573-6576.
[72]HARRISON J A, BRENNER D W. Simulated tribochemistry: an atomic-scale view of the wear of diamond[J]. Journal of the American Chemical Society, 1994, 116(23): 10399-10402.
[73]GAO G T, CANNARA R J, CARPICK R W, et al. Atomic-scale friction on diamond: a comparison of different sliding directions on (001) and (111) surfaces using MD and AFM[J]. Langmuir, 2007, 23(10): 5394-5405.
[74]YANG N, HUANG W, LEI D J. Control of nanoscale material removal in diamond polishing by using iron at low temperature[J]. Journal of Materials Processing Technology, 2020, 278: 116521.
[75]LIN Q, CHEN S L, JI Z, et al. High-temperature wear mechanism of diamond at the nanoscale: a reactive molecular dynamics study[J]. Applied Surface Science, 2022, 585: 152614.
[76]LIN J F, FANG T H, WU C D, et al. Nanotribological behavior of diamond surfaces using molecular dynamics with fractal theory and experiments[J]. Current Applied Physics, 2010, 10(1): 266-271.
[77]YANG N, ZONG W J, LI Z Q, et al. Amorphization anisotropy and the internal of amorphous layer in diamond nanoscale friction[J]. Computational Materials Science, 2014, 95: 551-556.
[78]LIU H Z, ZONG W J, CHENG X. Behaviors of carbon atoms induced by friction in mechanical polishing of diamond[J]. Computational Materials Science, 2021, 186: 110069.
[79]SHI Z Y, JIN Z J, GUO X G, et al. Interfacial friction properties in diamond polishing process and its molecular dynamic analysis[J]. Diamond and Related Materials, 2019, 100: 107546.
[80]SHI Z Y, JIN Z J, GUO X G, et al. Insights into the atomistic behavior in diamond chemical mechanical polishing with OH environment using ReaxFF molecular dynamics simulation[J]. Computational Materials Science, 2019, 166: 136-142.
[81]袁菘, 郭晓光, 金洙吉, 等. 金刚石化学机械抛光研究现状[J]. 表面技术, 2020, 49(4): 11-22.
YUAN S, GUO X G, JIN Z J, et al. Research status on chemical mechanical polishing of diamond[J]. Surface Technology, 2020, 49(4): 11-22 (in Chinese).
[82]YUAN S, GUO X G, LI P H, et al. Insights into the surface oxidation modification mechanism of nano-diamond: an atomistic understanding from ReaxFF simulations[J]. Applied Surface Science, 2021, 540: 148321.
[83]YUAN S, GUO X G, HUANG J X, et al. Insight into the mechanism of low friction and wear during the chemical mechanical polishing process of diamond: a reactive molecular dynamics simulation[J]. Tribology International, 2020, 148: 106308.
[84]YUAN S, GUO X G, MAO Q, et al. Effects of pressure and velocity on the interface friction behavior of diamond utilizing ReaxFF simulations[J]. International Journal of Mechanical Sciences, 2021, 191: 106096.


 手机资讯
手机资讯 官方微信
官方微信










 豫公网安备41019702003646号
豫公网安备41019702003646号